Introduction
Recombinant DNA technology has long been used to produce therapeutic insulin and, since most of the manufacturing cost (over 90%) of insulin is associated with recovery and purification, being competitive means maximizing the yield of high purity insulin. In addition, the purity requirements, as for any biopharmaceutical, are very stringent and are usually met by using high-resolution reversed phase chromatography (RPC) with silica-based resins. However, impurities from the feed (bioburden) often cause column fouling. The common method of cleaning-in-place using sodium hydroxide is limited for silica resins, so these impurities are difficult to remove. Introducing an upstream orthogonal purification step significantly reduces the burden on the expensive and high-performance RPC column, increasing the peptide yield and purity and also prolonging column life.
Results
The feed for the peptide purification process contained 72.5% pure human insulin precursor (Met-Lys-Human insulin), Figure 1A. Each purification step was optimized, and fractions were pooled to give a yield of 90% with highest possible purity.
A third purification step after the extract was applied to a column with WorkBeads 40S. A comparison with Capto SP ImpRes (GE Healthcare) as the capture step is shown in Table 1. A higher purity was observed by using WorkBeads 40S in the capturing step.
table 1. Purity comparison between WorkBeads 40S and Capto SP ImpRes for a pool yield of 90%.
The clean-up by CIEX purification minimized impurities to be applied on the second and third purification steps based on RPC. The same RPC column was used in both steps, while the conditions were different.
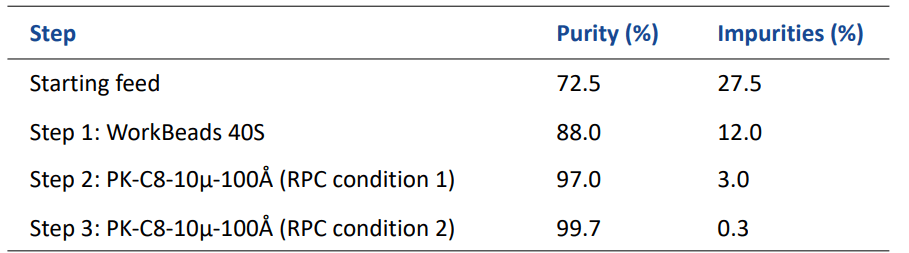
The optimized conditions and chromatograms are shown in Table 2 and Figure 1. The final purity of the three-step process was 99.7%. Table 3 shows the purity after each step in the process.
table 2. Three-step purification process of a recombinant human insulin precursor

figure 1. RPC analyses of purified pools from the optimized three-step purification. Feed (A), step 1: eluted pool from WorkBeads 40S (B), step 2: eluted pool from RPC1 (C) and step 3: eluted pool from RPC2, final product (D).
table 3. Purity obtained during the optimized three-step purification process of recombinant human insulin precursor
Conclusion and comments
By introducing a cation exchange chromatography (CIEX) step upstream of the high-performance silica-based RPC step, the burden from impurities was significantly reduced, from 27.5% to 12%. This improved formation minimizes the fouling of the RPC column, and the need for cleaning-in-place. The use of WorkBeads 40S can thus significantly extend the life-time of the subsequent RPC columns. The final purity for the process was 99.7%, well above the target of 99%.
WorkBeads 40S gave higher purity than Capto SP ImpRes, in the introduced CIEX capture step resulting in a more efficient capturing step and additionally also improved selectivity.