Subscribe to the blog
How reversing downstream bioprocess steps can make all the difference
Sep 4, 2023 9:00:00 AM / The Bio-Works Team
Improving the downstream bioprocess economy depends on fine-tuning an enormous range of parameters, from culture and harvesting to purification and final polishing. Success depends on the ability to troubleshoot difficulties and identify novel approaches that will boost process efficiency in a cost-effective manner. This is particularly true when high-value chromatography columns are used in the process.
Bioburden and cleaning-in-place procedures can reduce column lifetime and capacity, and threaten product quality and yield. An alternative approach that protects these valuable columns is to reverse the first two steps to remove the potentially damaging components of a feedstock before proceeding with highly selective capture followed by polishing.
Troubleshooting your process
Building insight into the challenges of process development and the solutions available is a key component of successful process development. The Critical Quality attributes (CQA) and Critical Process Parameters (CPP) for the process should be properly defined and analytical tools must be available to enable thorough monitoring of the process.
Warning signs that a process is running out of control can include:
- Low or falling yields of active, correctly folded protein
- Increase in impurities such as host cell proteins (HCPs), host cell DNA (HCD), specific proteases or lipids
- Increase in backpressure in columns
- Decrease in column efficiency and asymmetry
- Decrease in column lifetime
Reduced yield of the product and increasing levels of HCPs can be related to each other, especially if the HCPs include proteases that can degrade the product. Increasing backpressure or decreasing the column efficiency and asymmetry may be a result of the accumulation of biomolecules that foul or degrade columns. Fouling is a cumulative process that may block pores and/or block binding sites on the surface of the resin, reducing mass transfer into the resin bead, and can be followed by checking several indicators. These include:
- The measurement of the uniformity of column packing (plate height, asymmetry)
- The chromatographic profile of the process
- The product yield
- The product quality
Fouling can be influenced by interactions between deposited materials and changes in conditions or steps, such as cleaning protocols. To examine these problems in more detail, let us take the purification of antibodies using protein A columns as an example.
A case in point: Protein A column chromatography
Monoclonal antibody (mAb) production is a significant part of the biopharmaceutical industry, and protein A affinity chromatography is central to the robust manufacture of these products. Protein A columns can be very expensive, comprising up to 50–80% of total resin costs in some cases, and more advanced and highly selective protein A columns are used in the first step of purifying high titers of IgG feedstock material. Subsequent polishing with anion exchange, cation exchange, and hydrophobic interaction chromatography removes problematic, co-purifying HCPs and HCD. This widespread approach puts protein A columns on the front line regarding exposure to bioburden. To be economically viable, protein A columns must be cycled hundreds of times despite being sensitive to cleaning-in-place (CIP) procedures that commonly involve sodium hydroxide, and some fouling components may be virtually impossible to remove even with harsh CIP methods. Improving protein A column performance and resin lifetime has become a significant focus for downstream process R&D.
Many factors can reduce the performance of protein A column resin, including:
- Ligand leaching or degradation
- Occlusion of the pores resulting in a reduction in available surface area or deterioration of the packed bed from resin damage
- Proteases that cleave the protein A ligand
- Non-specific binding of released protein A to the IgG, resulting in co-elution with the antibody
- Dissolution of the base matrix during pH cycling due to chemical breakage of the glycosidic linkages in the agarose matrix component
The performance of protein A columns is commonly monitored by measuring dynamic binding capacity (DBC), which describes the amount of sample that will bind to a resin packed in a column under defined conditions. DBC is highly dependent on operating conditions, sample preparation, and sample origin. A survey of 15 major manufacturers of mAb products showed that 80% of respondents saw a decrease in DBC and flattening of the slope of the breakthrough curve as the cycle number increases or a reduction in DBC with a sharpening of the slope of the breakthrough curve as the cycle number increases[1]. The respondents also saw resin discoloration and eluate peak broadening.
The survey also showed that the two most significant indicators of sub-optimal resin performance are the level of HCP in the pool and specific proteases. Other significant effects include the level of leached protein A ligand in the collection, DNA concentration, and the amount of lipids.
The problem of fouling has been put into clear graphic terms by Lintern and coworkers, who have shown that there is a correlation between the appearance of surface deposits (foulants), reduction in resin capacity, and the increase in the number of host cell proteins carried over between cycles of agarose-based protein A chromatography (Fig 1; references 2, 3).
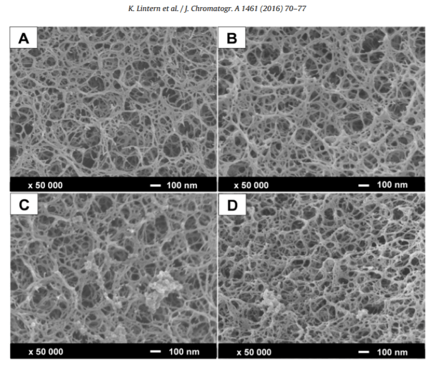
Figure. 1. Fouling of a protein A resin. Scanning electron microscopy images at 50 000 × magnification of the surface of MabSelect SuRe™. A) Unused resin, B) after 50 cycles, C) 60 cycles and D) 100 cycles. Taken from Figure 4, Lintern et al, 2016 [open access article under the CC BY license]
Observations such as these have stimulated the development of methods for the direct, quantitative assessment of fouling that could be of great interest to the biopharmaceutical industry[3].
Protection boosts process efficiency
Several strategies have become established for downstream purification. As we have seen, one technique for purifying mAbs involves exposing the most expensive component in the production of a dominant sector of biopharma, the protein A column, to excessive bioburden and rigorous CIP procedures.
The question we must ask ourselves is, can we protect this key component from damage while maintaining and improving process efficiency and economy?
There is indeed an approach that enables the early rapid removal of HCP impurities, including proteases, which can foul columns and even degrade the product.
First, a little background:
A strategy originally formulated by Bio-Works focused on how and which complementary separation techniques could be combined, and sample manipulation reduced to a minimum. These three purification process phases (CEP) focus on (i) concentrating the target substance and removing harmful impurities (Capture), (ii) further removal of bulk impurities (Enhance), and finally, (iii) removal of remaining trace impurities and unwanted structural variants of the target for example aggregates (Polish). Purification should require no more than three chromatographic steps. If there are more, too much target protein will be lost, and the ratio of purity to cost becomes unacceptable.
This strategy can now be modified by changing the order of the steps, with significant benefits to both purity and process performance.
An initial, preferably orthogonal, purification step (Enhance) removes a significant portion of impurities such as host cell DNA, HCPs and endotoxins. Early removal of these impurities eliminates bioburden on the next step (Capture) involving a valuable resin and should thus extend its lifetime. The process is completed with the Polish step.
The usefulness of this approach has already been demonstrated.
For example, in the case of the purification of mAbs that we described above, affinity chromatography with WorkBeads™ 40 TREN is used upstream of protein A affinity chromatography to protect the protein A column and reduce the need for CIP.
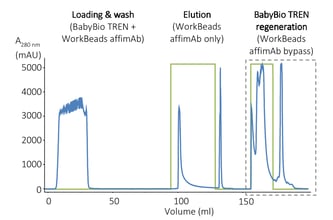
You can find the details for the figure above in our application note: Protection of Protein A resin during mAb purification.
Another example is the use of cation exchange chromatography upstream of high-value reversed-phase chromatography (RPC) columns to improve yield, purity and process time. In this test, WorkBeads 40S proved superior to other resins for this purpose.
Learn more about how you can improve mAb purity and prolong the life span of protein A columns in this article here.
References
- Re-use of Protein A Resin: Fouling and Economics, Pathak, M et al. BioPharm International, Volume 28, Issue 3, 2015
- Residual on column host cell protein analysis during lifetime studies of protein A chromatography, Lintern, K. et al. J Chromatog. A, 1461 (2016) 70–77
- Pathak, M. et al. Fluorescence based real time monitoring of fouling in process chromatography. Rep. 7, 45640; doi: 10.1038/srep45640 (2017)