Subscribe to the blog
New biopharmaceuticals on the horizon: A purification challenge
May 31, 2023 10:18:37 AM / The Bio-Works Team
In 1982 a new type of pharmaceutical appeared on the market. Recombinant human insulin was approved for human therapeutic use and is generally acknowledged as the first “biopharmaceutical” or “biologic.” Biopharmaceuticals have made enormous progress since then, to the point where they have now begun to dominate the pharmaceutical pipeline.
Forty years later, in 2022, biopharmaceuticals approvals* nudged ahead of small molecules for the first time ever, accounting for about a third of 2022’s approvals. This was when the number of new drug approvals in general in the US was actually about 25% reduced, to the lowest level since 2016.
*(e.g. for antibody–drug conjugates, bispecific proteins, cell and gene therapies)
This glorious biopharmaceutical revolution has, of course, brought new challenges, such as the cost.
For example, in 2019 we wrote about the price pressures associated with biopharmaceuticals:
"The consequences of these pricing pressures have led to the need for more specific, robust purification techniques that can drive down the cost of biopharmaceutical production and bring more affordable drugs to patients to increase the quality of life."
Now the revolution is taking off in new directions and stirring up a whole new set of challenges.
Drug developers have learnt how to combine, derivatize and modify the original biopharmaceutical molecules to produce a whirlwind diversity of new and better-targeted therapeutics.

There are even newer modalities coming up fast behind them: Viral vectors, Adeno-Associated Virus (AAV), Lipid Nano Particles (LNP), mRNA, and plasmid DNA — in many cases bearing the promise of therapies where there were previously none.
 In fact, new types of biopharmaceuticals, including antibody-drug conjugates, bi-specific antibodies, and cell and gene therapies, have reportedly already accounted for about one-third of last year’s FDA drug approvals in the US.
In fact, new types of biopharmaceuticals, including antibody-drug conjugates, bi-specific antibodies, and cell and gene therapies, have reportedly already accounted for about one-third of last year’s FDA drug approvals in the US.
This tide of diversity in biopharmaceuticals rides on several waves, including advances in technology, a better understanding of disease mechanisms, and the increasing demand for personalized medicine.
Examples of the diversification in next-generation biopharmaceuticals on the horizon:
Biopharmaceuticals are being designed that specifically interact with a particular molecular target. This allows for more effective treatments with fewer side effects. For example, many cancer treatments are now targeted to specific molecules involved in cancer growth. One example is the next-generation T-cell receptor therapies (TCR-Ts) with fully individualized TCR therapies as well as TCR therapies targeting, for example, mutated KRAS and mutated TP53.
Another example of this is Antibody–drug conjugates (ADCs) which combine the high specificity of antibodies with cytotoxic small molecules to deliver highly potent payloads within the targeted cell. These ”weaponized antibodies” can significantly improve the efficiency of cytotoxic molecules and reduce their off-target toxicity, a major issue of conventional cytotoxic chemotherapies. This family has evolved into a mature component of the anticancer pharmacopeia (with 13 agents currently approved for the treatment of cancer).
Bi-specific and multi-specific antibodies are antibodies designed to recognize and bind to multiple targets simultaneously. They can be used to treat diseases such as cancer and autoimmune disorders.
Peptides are now being used in gene therapy to deliver gene therapies to specific cells in the body. Peptide-based carriers can protect the gene therapy from degradation and facilitate its delivery to the target cells. These therapies have the potential to treat a wide range of genetic disorders, such as hemophilia and sickle cell anemia.
Oligonucleotides are being modified in many ways to bring about improvements in stability, pharmacokinetics and efficacy. For example, oligonucleotides such as siRNA or miRNA, used for RNA interference (RNAi), can be designed to bind to specific mRNA molecules and trigger their degradation, leading to inhibition of gene expression. And peptides are being conjugated to oligonucleotides, combing these two powerful tools.

Particularly in the well-established mAb area, there are big changes afoot
Antibody manufacturing has evolved over decades to become a stable process with, for example, cloned, standardized cells producing the antibody and a standard method for purification of the drug substance. Methods are so well-developed that variability has primarily been due to biological variations.
But not anymore…
Over the last 6 or 7 years, monoclonal antibody (mAb)-based pharmaceuticals have evolved significantly with some of the most notable changes being the development of bispecific antibodies and antibody-drug conjugates (ADCs) as well as the increased use of biosimilars.
Downstream purification is racing to keep up
With biopharmaceuticals becoming more numerous, diverse and complex, the downstream purification processes for isolating and purifying biologic drugs are facing some real challenges in keeping up.
One example comes from the mAb field and bispecific antibodies, where the challenge is to separate the drug which has two targets from its variants that have a single target. The usual result: lower yields and reduced purity.
For a long time, biopharmaceuticals have essentially been proteins. Full stop. Proteins are variable enough to have kept a whole industry busy all this time, but they still resemble each other much more than they resemble mRNA molecules for example, or viral particles, or plasmid DNA.
Until new processes and tools for downstream purification can be developed, people are forced to start with a current off-the-shelf product developed for proteins and try to adapt it for use with an mRNA, or a plasmid. Like using one screwdriver for all types of screws; it often works, but it could be better.
To address these challenges, new purification methods will need to be developed that can handle the increasing diversity of biologic drugs. With many interested parties, powerful driving forces, and the race to reach the market before the competitors, this is likely to move quite rapidly. But it is of little comfort, of course, to those that have already worked their way through all the resins on the market without achieving their purification targets.

There is some light at the end of the tunnel, however. With experience in creating and modifying chromatography resins, it is possible to modify several parameters of the resins using knowledge of how each can affect the final results.
For example, the agarose-based chromatography resins, called WorkBeads, that Bio-Works produce can be modified in several ways.
A resin, depending on the area of use, may be composed of the base matrix (the bead), a linker arm and a ligand.
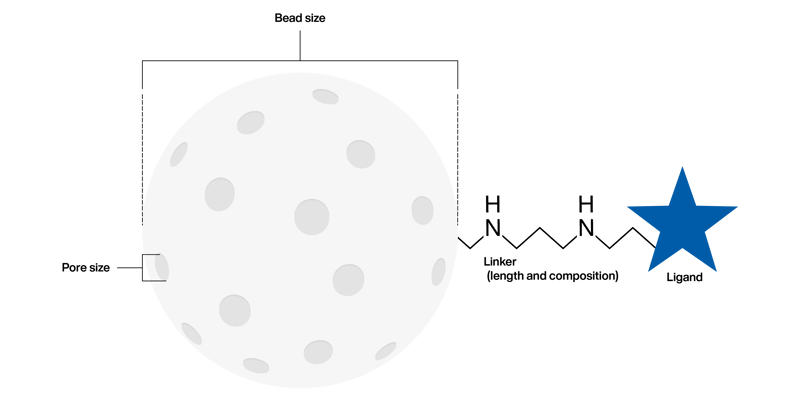
Each of these can be adjusted in the following ways:
Bead size
- Large – for a capture step (> 65 µm)
- Mid – for an intermediate step (40 ~ 65 µm)
- Small – for a polish step (< 40 µm)
Pore size (Globular protein exclusion limit)
- 100 kDa cut-off
- 1000 kDa cut-off
- 10000 kDa cut-off
- 30000 kDa cut-off
Linker
- Length
- Atomic composition
Ligand
- Nature of ligand
- Concentration of ligand immobilized on bead/base matrix
Changing one or more of these parameters could make the difference between being able to purify a target molecule or not. But it could also produce an incremental change that suddenly makes a process economically feasible.
For example, a process can be improved by introducing a very specific and efficient capture step so that less polishing is needed and the cost is lowered.
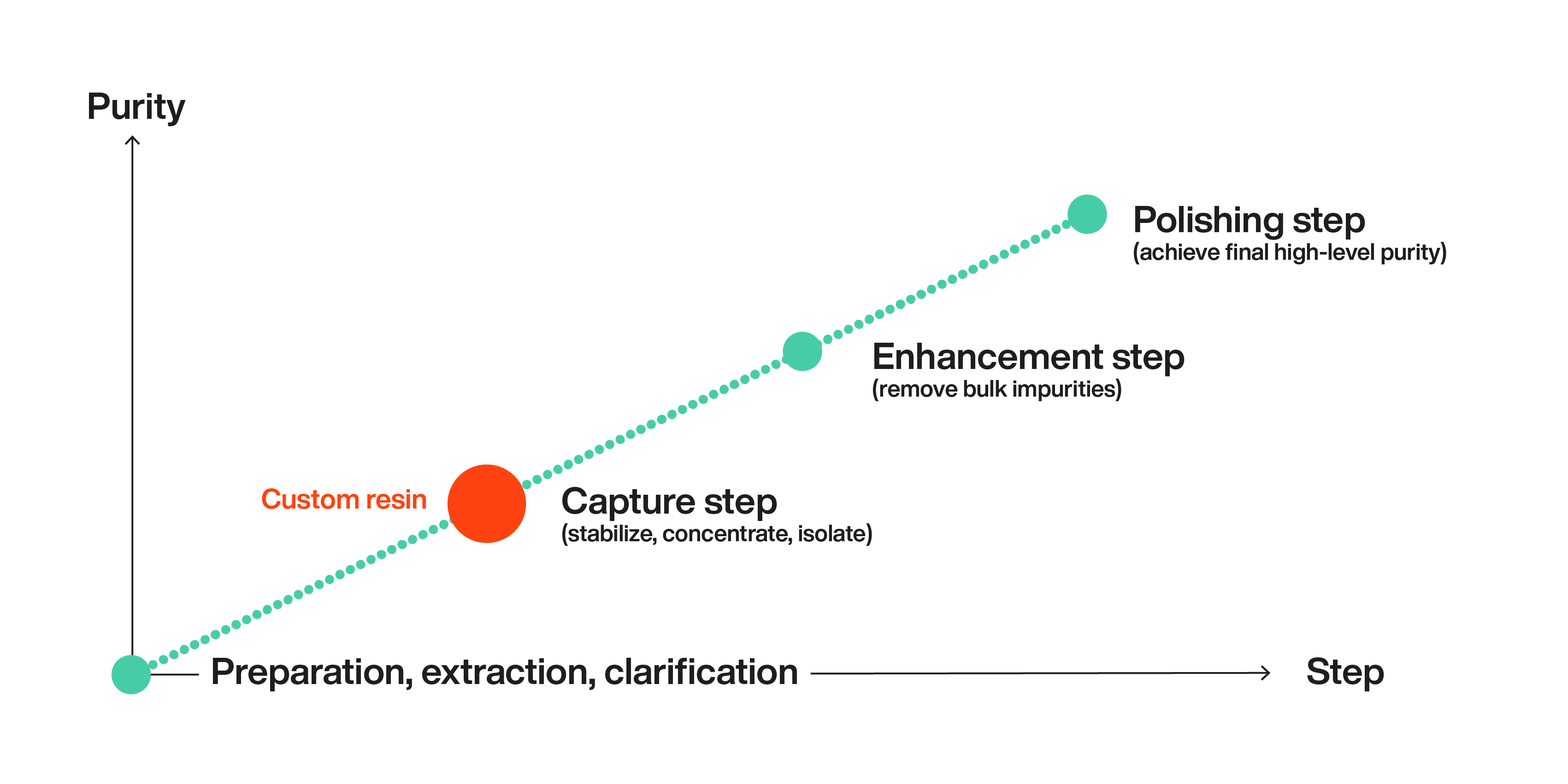 The more we investigate the possibilities, the more we all learn.
The more we investigate the possibilities, the more we all learn.
Do you have a specific purification challenge that you need help with? Are you curious to know whether a custom resin is the solution?